Autoclaving. Containers are positioned in an autoclave and subjected to superior-stress steam to get rid of microbes.
Key phrases: high-quality administration program; good quality danger management; FMEA; GMP; filling process; rubber stopper washing
Place force differentials needs to be monitored on an ongoing foundation. Walls, flooring and surfaces ought to be subject to some pre-identified application of cleaning and disinfection.
Total, aseptic processing takes place in 3 ways that ensure the sterility of solutions as well as ecosystem, as noticed underneath.
On top of that, the flexibility of liquid bottle filling equipment allows brands to adapt immediately to various liquid types and bottle measurements. Regardless of whether it’s liquid prescription drugs, syrups, or answers, these devices can effectively tackle distinct viscosities and packaging demands.
Note: No volume of shell out is regarded as wages or payment till these total is gained, vested, and determinable. The quantity and availability of any bonus, commission, incentive, benefits, or any other type of compensation and Rewards that are allocable to a particular employee continues to be in the organization's sole and absolute discretion Except if and until eventually paid out and could possibly be modified at the Company’s sole and absolute discretion, consistent with relevant legislation.
Making sure there aren't any present microbes in the surroundings that can influence the integrity of merchandise in advance of final sealing and packaging via environmental checking and concluded product or service testing.
I am able to revoke my consent Anytime with result for the longer term by sending an e-mail to unsubscribe@sartorius.com or by clicking around the "unsubscribe" backlink in e-mails I've received.
Spouse and children owned & read more operated for fifty+ years– we place customer service and integrity at the center of all the things
The guidance provided by all system homeowners and supervisors is significantly appreciated for the valuable opinions and brainstorming sessions supplied by in defining risks while in the cleanroom entry and exit techniques, glass bottle washing equipment operation, and glass filling approach.
Include Mycap® to bottles and carboys utilized for freeze/thaw operations to significantly improve filling and draining by creating each an aseptically closed process and liberating the process within the confines of biosafety cupboard.
Parenteral items are medications implanted or injected instantly through the skin to enable direct administration into tissue organs, blood vessels, or lesions.
When building a sterile merchandise, folks normally usually do not recognize what’s required to manufacture the products. Does the molecule need aseptic filling, or can it's terminally sterilized?
A “media fill” (occasionally generally known as a “process simulation”) may be the general performance of the aseptic manufacturing procedure using a sterile get more info microbiological development medium in place of the drug Remedy.
 Ralph Macchio Then & Now!
Ralph Macchio Then & Now! Devin Ratray Then & Now!
Devin Ratray Then & Now!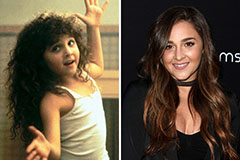 Alisan Porter Then & Now!
Alisan Porter Then & Now! Kelly Le Brock Then & Now!
Kelly Le Brock Then & Now! McKayla Maroney Then & Now!
McKayla Maroney Then & Now!